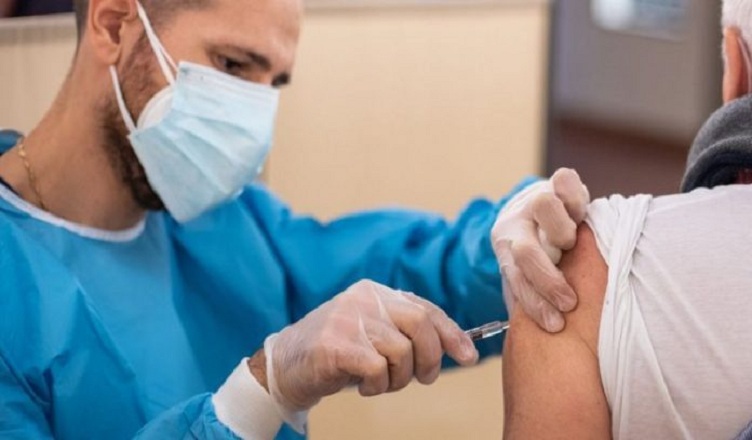
(COLOMBO, LANKAPUVATH) – major trial of a vaccine to protect against Covid-19 has launched in the UK – the third such trial in the country.
The jab – designed by the Belgian company Janssen – uses a genetically modified common cold virus to train the immune system.
It comes a week after preliminary results showed another vaccine offered 90% protection.
However, many types of vaccine are likely to be needed to end the pandemic.
The success of the vaccine developed by Pfizer and BioNTech has caused global excitement. However, it has not yet been approved for use and we still do not know how well it works in the elderly or how long immunity lasts.
The hunt for Covid vaccines continues as a different approach may yet be better, or better in some age groups, and one company will struggle to immunise the planet.
“It is really important we pursue many different vaccines from many different manufactures,” said Prof Saul Faust, the director of the NIHR Southampton Clinical Research Facility, who will run the trial.
He added: “We just don’t know how each of these vaccines is going to behave and we can’t be certain vaccine supply will be efficient and secure from one manufacturer.”
The trial has started the job of recruiting 6,000 people in the UK. Other countries will join the effort to bring the total up to 30,000.
Half of the volunteers will be given two doses of the vaccine around two months apart.
Janssen already has one large scale trial of its vaccine in which volunteers get one dose. This trial will see if two gives a stronger and longer lasting immunity.
It could take six to nine months before the results are available.